Projects
Industrialization of an oxygenator for biomedical
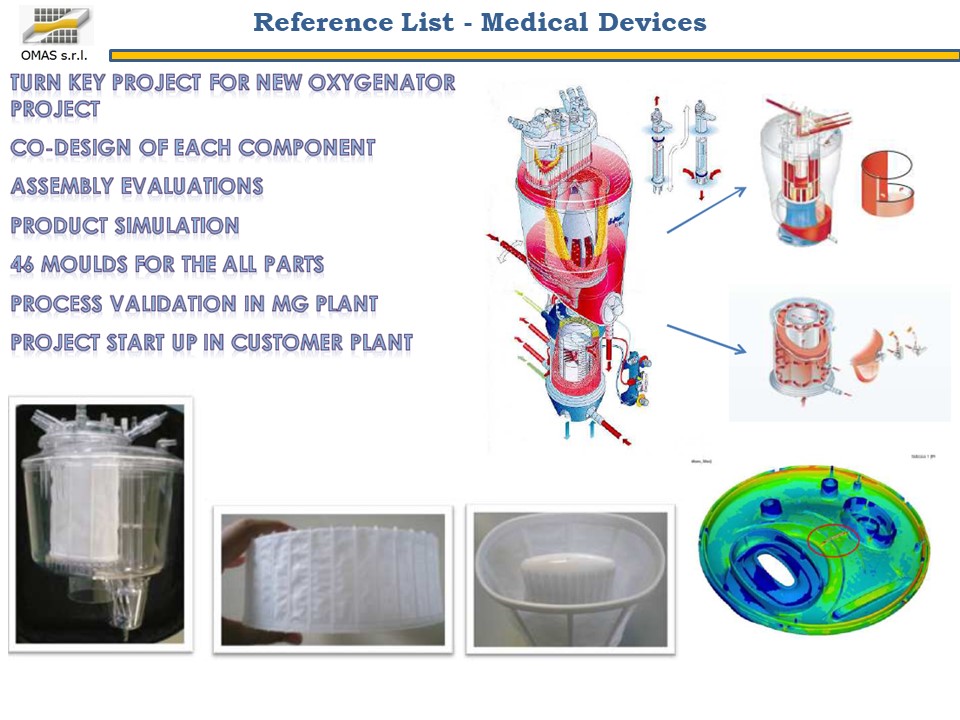
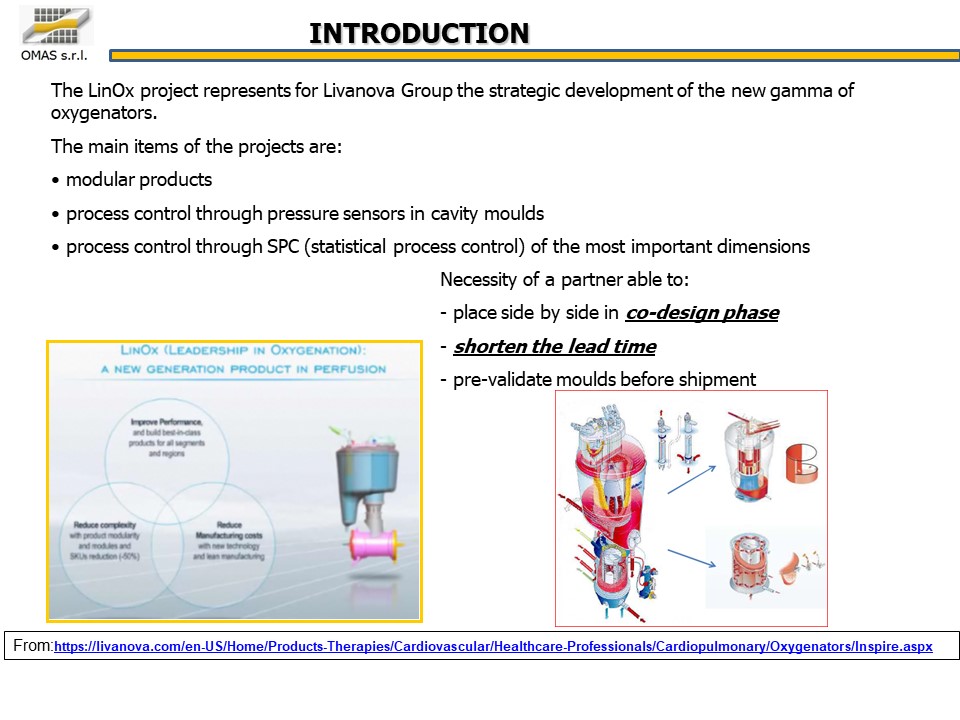
In the biomedical world the attention to quality and the search for competitiveness go hand in hand: these two elements have established the guidelines for a project aimed at producing 40 different details of an oxygenator for cardiac surgery, to be produced using the best available implants.
Objective:
- Industrialize the 40 components of an oxygenator for cardiac surgery by optimizing the use of existing injection presses.
- Validate the product and process prior to equipment transfer.
- Time guarantee on the total project
Activity:
- Product / process optimization (printability, criticality elimination).
- Identification of alternative mold configuration scenarios.
- Mold construction.
- Product and process certification.
Product / process optimization and industrialization
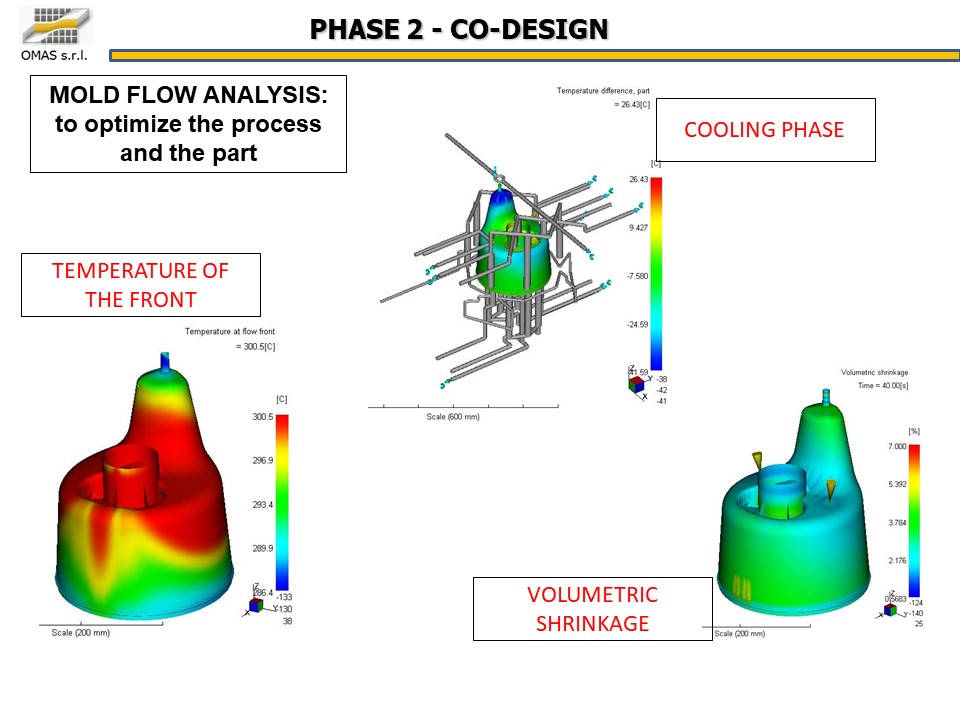
The absolute precision of the products in terms of geometry and dimensional stability is a key factor in such a delicate sector as biomedical.
Working in advance on the geometry of the products to widen the molding window and thus make the process more reliable was a fundamental element for the success of the project.
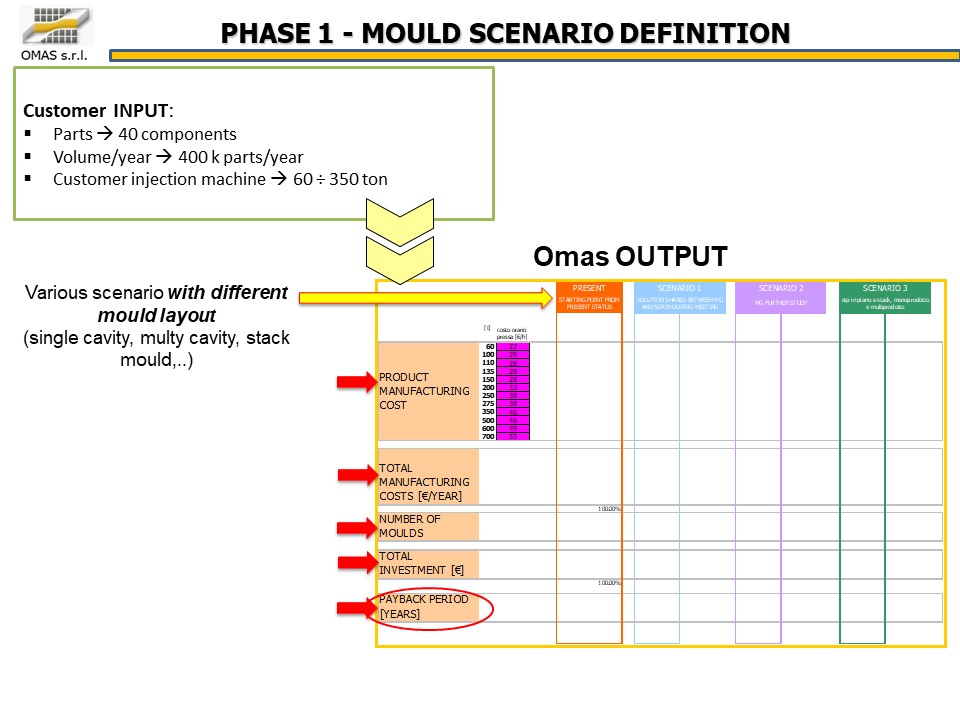
The customer’s need was to produce 400,000 oxygenators per year, each product being made up of 40 PC parts to be produced on a fleet of presses with a tonnage of 60 ÷ 350 tons.
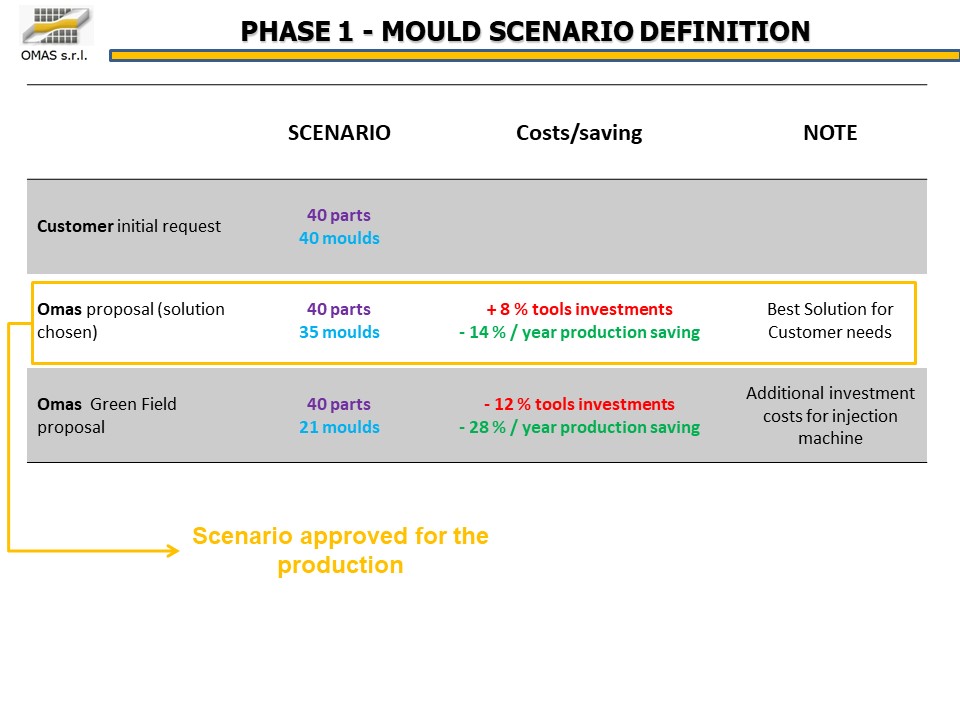
Various configurations (multi-cavity book or flat molds) were analyzed from a technical / economic point of view, finally choosing the most efficient one compared to the available presses: 35 molds for the production of 40 components.
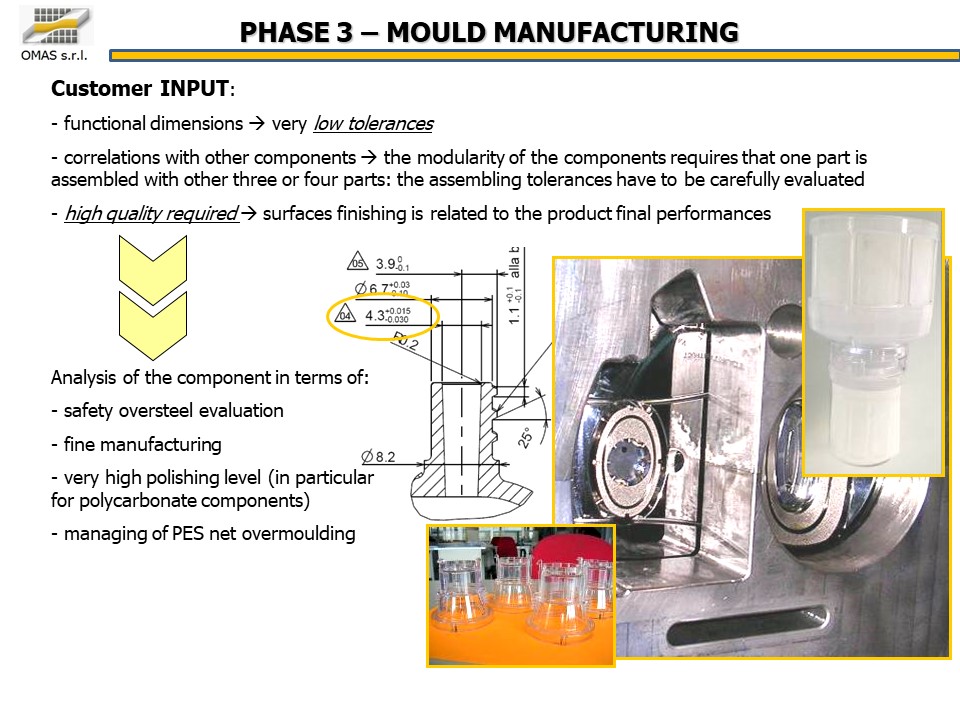
Importance of quality
After identifying the configuration of the 35 molds, the focus shifted to their quality, considering that in this case it is strongly correlated to the functionality of such a delicate product. The accuracy of the processing to ensure the cleanliness and precision of the surfaces and the dimensional stability of the interface areas were a key success factor of the project.

Validation and approval
The validation procedure in required:
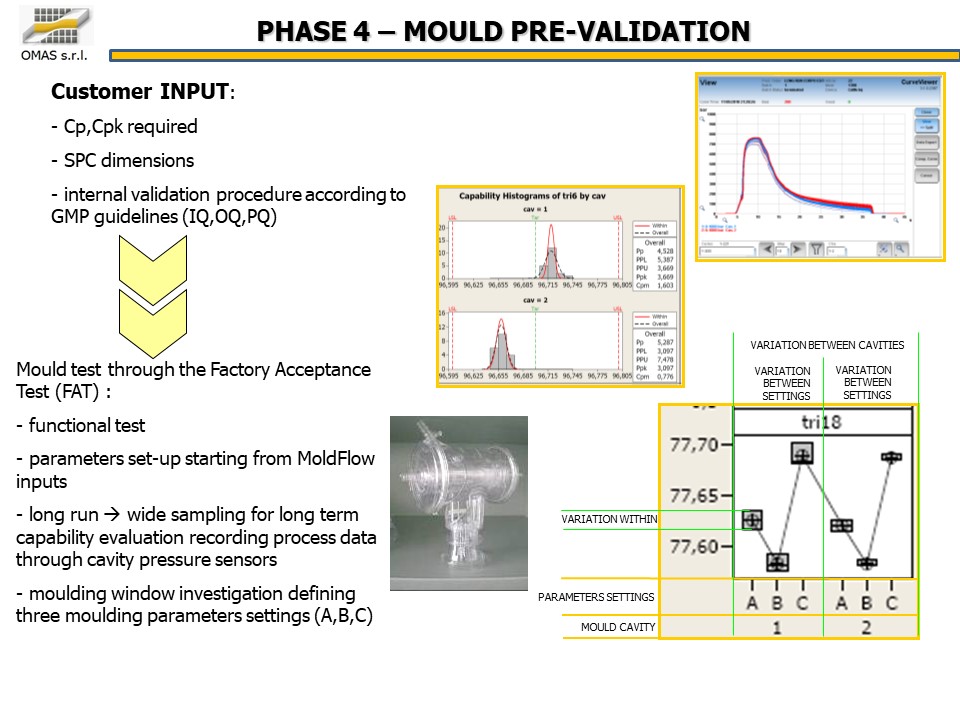
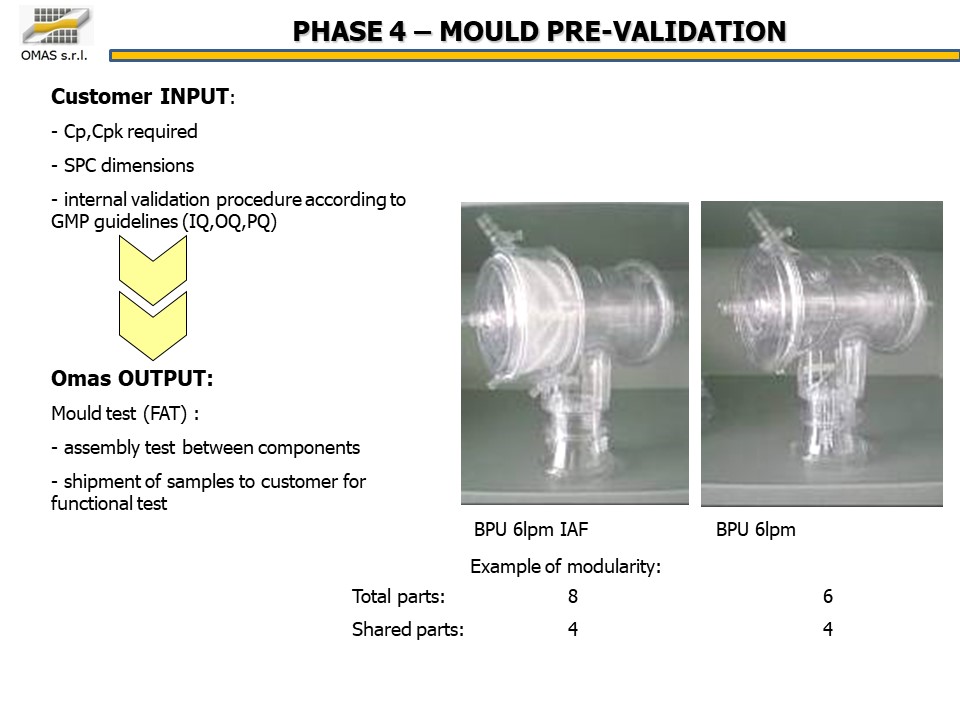
- Cp, Cpk required
- SPC dimensions
- validation according to GMP (IQ, OQ, PQ)
For each mold were made:
- Mold test through the Factory Acceptance Test (FAT):
- functional test
- parameters set-up starting from MoldFlow inputs
- long run à wide sampling for long term capability evaluation recording process data through cavity pressure sensors
- molding window investigation defining three molding parameters settings (A, B, C)
Accuracy and speed
Despite the delicacy of the project and the high accuracy required in each phase, the coordination as the customer’s sole partner has made it possible to move from project start (feasibility analysis) to SOP at the production plant in just 15 months.


 Sito in Italiano
Sito in Italiano Sitio en Español
Sitio en Español